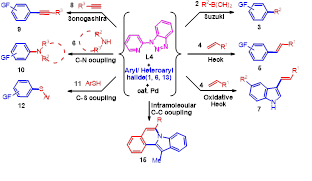
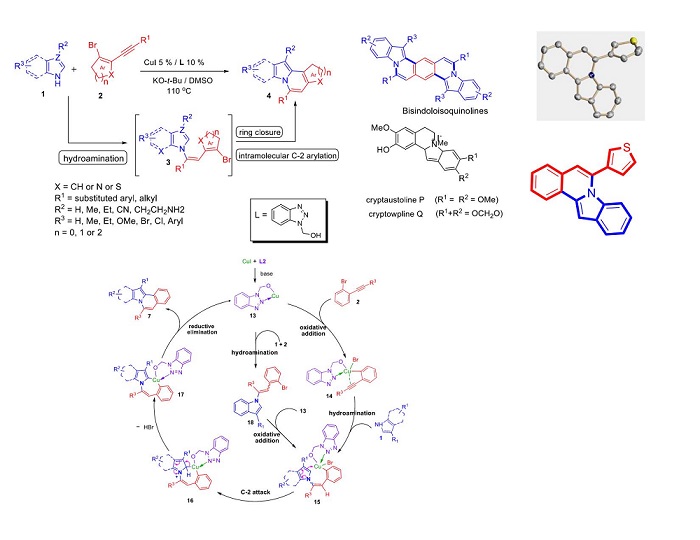
Based on the above results we have designed a novel strategy for the copper-catalyzed tandem synthesis of indolo- and pyrrolo[2,1-a]isoquinolines (core nucleus of natural product, Cryptaustoline and Cryptowoline ) from ortho-haloarylalkynes by sequential intermolecular addition of N-heterocycles onto alkynes, followed by intramolecular ring closure by C-2 arylation. This chemistry involve the preferential nucleophilic addition of indoles and pyrroles onto the ortho-haloarylalkynes over N-arylation of the aryl halide. The proposed mechanism was confirmed by various controlled experiments and X-ray crystallographic studies. Developed novel chemistry can allow direct access of various types of diversely substituted N-heterocycles, carbocycles, natural- products-like compounds, synthetic drugs and π-conjugated organic materials. We proposed two possible routes for the generation of key intermediate 15. i. via oxidative addition followed by hydroamination of intermediate 14. ii. via hydroamination to form enamine intermediate 18 and followed by the oxidative addition.



Scientific Contribution
1. Discovery of benzotriazole and its derivatives as efficient and inexpensive ligands for the coupling reactions and their application in the tandem synthesis of heterocycles/natural products/organic materials
Benzotriazole has been much explored by the Katritzky group as a synthetic auxiliary in a number of transformations due to its interesting properties. We have first time noticed that this air and moisture stable molecule ‘benzotriazole’ has excellent coordination capability which could be favorable for stabilizing catalytic species and assisting the catalytic cycle. We started our journey by using benzotriazole as Ligand for the copper-catalyzed C-N and C-S coupling reaction. Encouraged by preliminary results we have designed a large number of benzotriazole based ligand for the various coupling reactions and observed the designed ligand (hydroxymethyl)benzotriazole was more efficient than benzotriazole for C-N coupling reaction.
Novel chemistry being developed in our laboratory using designed ligand:
Angew. Chem Int. Ed. 2009, 48, 1138-1143
Conformation of the Proposed Mechanism
Org. Lett. 2011, 13, 1630-1633, J. Org. Chem. 2012, 77, 5633-5645
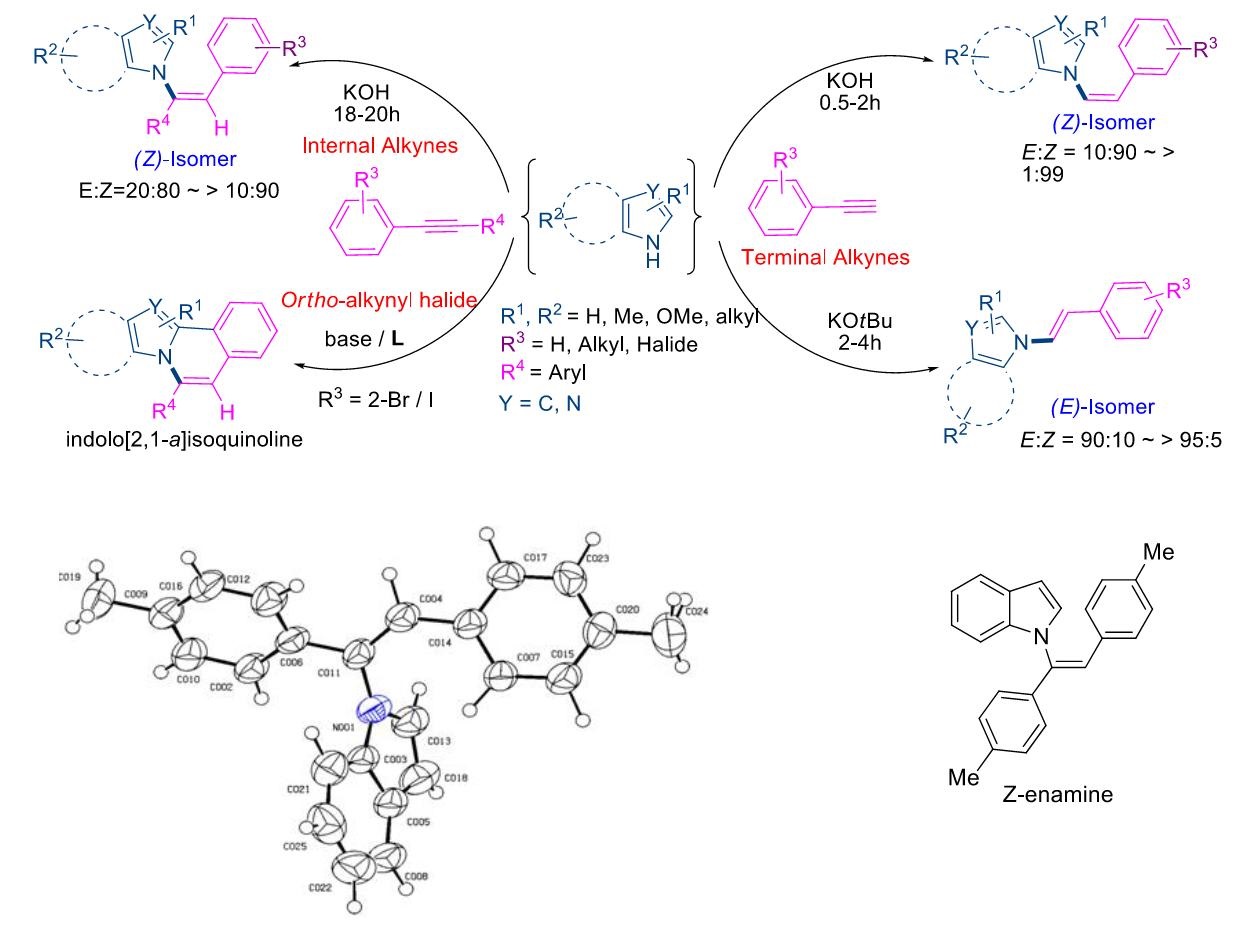
Regio- and stereoselective addition of N-heterocycles to alkynes using KOH was performed and it was found that, Formation of (Z)-isomers and its conversion in to (E)-products was found to be dependent upon time as well as choice of the base. Selective attack of N-heterocycles on more electrophilic alkynyl carbon and stereochemistry of the products was confirmed by the DFT calculations, X-ray crystallographic studies and intramolecular cyclization of ortho-haloalkynes in to indolo-[2,1-a]isoquinolines. This study supports the formation of indolo/pyrrolo[2,1-a]isoquinolines via Z-enamine.
Org. Lett. 2012, 14, 1106-1109.
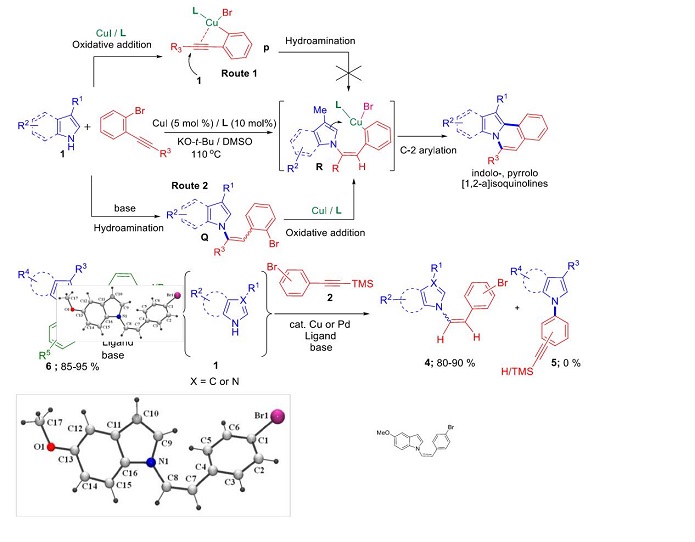
Reaction of various N-heterocycles and halo-substituted arylalkynes was performed and it was observed that hydroamination is preferred over amination of aryl halide. The results of the present study, preferential addition of N-heterocycles onto halo-substituted arylalkynes suggests that the mechanism of the copper-catalysed tandem synthesis of indolo- and pyrrolo[2,1-a]isoquinolines proceeds via generation of intermediate Q through hydroamination followed by oxidative addition to the key intermediate R and not vice versa (Scheme 2, route 2).
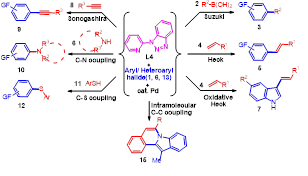
Synthetic application: Synthesis of 1,6-Naphthyridines, bisindolo-, and pyrrolo[2,1-a]isoquinolines
J. Org. Chem. 2012, 77, 8191–8205
We have successfully extended the scope of the developed chemistry for the regioselective tandem synthesis of biologically importantNaphthyridines and bisindolo[2,1-a]isoquinolines, a regioisomer of bisindolo[2,1-a]quinolines used as single-crystal field-effect transistor
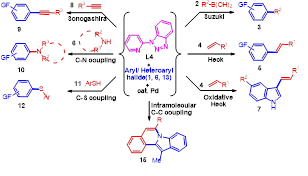
2-(1-Benzotriazolyl)pyridine (BtPy): A Novel Inexpensive and Robust Ligand for the Palladium-Catalyzed C-C (Suzuki, Heck, Oxidative-Heck, Sonogashira), C-N and C-S Coupling Reactions:
Tetrahedron Lett. 2007, 48, 4207-4210; Tetrahedron Lett. 2007, 48, 7199-7202; Tetrahedron 2009, 65, 8434-8439; Advances in Heterocyclic Chemistry 2012 , 107, 103-132; Adv. Syn. Cat. 2013, 355,421-438
In continuation of our work on the designing of benzotriazole based ligands for the coupling reactions, recently we have designed an N,N type phosphine free, air stable and robust ligand BtPyby incorporating pyridine ring at N-1 position of the benzotriazole. Results of using this ligand are very interesting and significant. We have first time observed that designed ligand BtPy efficiently catalyzed the Suzuki, Heck, Oxidative-Heck, Sonogashira, Buchwald-Hartwig (C–N), and C–S coupling reactions.
2. Diversity Oriented Synthesis (DOS) of Over Hundred Natural-Product-Likes and π-Conjugated Scaffolds: A Novel Cascade Reaction
Green Chem. 2011, 13, 1640-1643; Eur. J. Org. Chem. 2012, 4590-4602
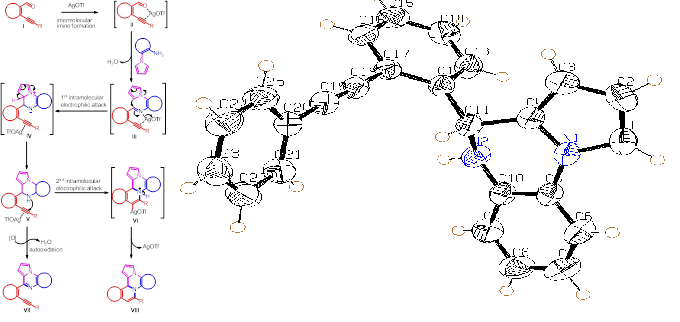
Design and synthesis of biologically relevant, drug-like small molecules to perturb and analyze biological systems is one of the main challenges in the medicinal chemistry. Diversity Oriented Synthesis (DOS) of small molecules is new algorithm that enables efficient synthesis of complex molecules. This is one of the most challenging ongoing projects of our laboratory by using electrophilic cyclization chemistry.
In this project we have designed a novel cascade synthetic strategy for the “Diversity Oriented Synthesis (DOS) of Over Hundred Heterocyclic/Natural-Product-Likes and π-Conjugated Scaffoldsstrong>”. Strategy involves the construction of designed scaffolds by the reaction of ortho-akynyaldehydes with appropriate amines/nucleophiles under silver-catalysis by the sequential i) intermolecular C-N bond formation; ii) followed by two intramolecular C/N/O/S-C (attack of nucleophile on imine carbon: intermediate III) and N-C (attack of nitrogen on activated alkyne: intermediate V) bond formation. The mechanism of the designed reaction is well established by the spectroscopic and X-Ray crystallographic studies of the isolated intermediates III, V and the final product VIII.
We have successfully synthesized more than 50 distinct heterocyclic scaffolds (>350 distinct novel compounds). It is important to mention that above 25 scaffolds (> 100 novel molecules) were synthesized in water using AgNO3 as a catalyst. The scope of the developed chemistry was successfully extended for the synthesis of sterioselective and diastreoselective molecules. This developed process is expected to find application in organic synthesis/medicinal chemistry/material science in general, and in the construction of a variety of interesting compounds. The preliminary results are very exciting and interesting. Preliminary in-vitro screening results of some scaffolds on cancer cell lines are very impressive.
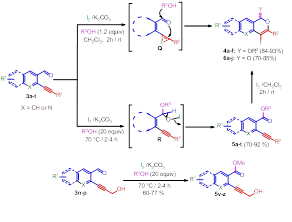
3. Iodine-mediated chemoselective direct oxidative esterification of aldehydes without affecting alkynes and 10 alcoholic groups: New addition in the functional group transformations
J. Org. Chem. 2010, 75, 7691-7703, Chem. Commun, 2010, 46, 4064-4066, ACS Comb. Sci. 2011, 13, 530-536
This is another interesting and practically useful novel chemistry being developed in our laboratory. This developed process provides a novel access for the chemoselective synthesis of esters from aldehydes without oxidizing/affecting the primary alcoholic and alkyne group present in the substrate via formation of hypoiodide intermediate. Developed oxidative esterification process, provides a powerful tool for the synthesis/preparation of wide range functionalized pyranoquinolinones, isocoumarins, α-pyranones and natural products. Process is a useful addition in the organic functional group transformations where protection and deprotection is required.
Note: This chemistry has been successfully implemented in the M.Sc practical as a green practical.
4. Site-selective electrophilic cyclization and subsequent ring opening: An efficient route to pyrrolo[1,2-a]quinolines and indolizines
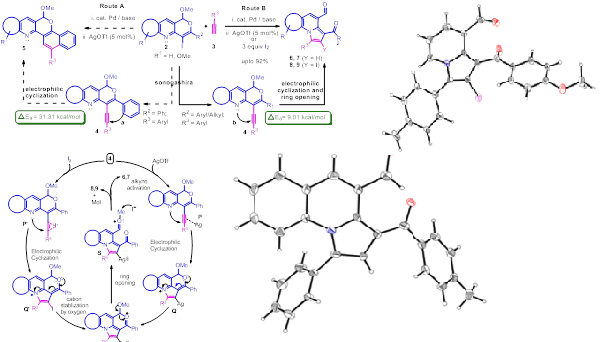
J. Org. Chem. 2012, 77, 8562–8573
An efficient strategy for the synthesis of pyrrolo[1,2-a]quinolines and indolizines from pyranoquinolines via site-selective electrophilic cyclization and subsequent opening of pyran ring using silver/iodine under mild reaction conditions is described. This approach involves preferential attack of pyridyl nitrogen over aryl ring and leads to the formation of 5-endo-dig cyclized products. Quantum chemical calculations between C-N (ΔEa = 9.01 kcal/mol) and C-C (ΔEa = 31.31 kcal/mol) bond formation were performed in order to rationalized the observed site selectivity. Structure of the products was confirmed by X-ray crystallographic studies. Iodine substituted compounds generated by the electrophilic iodocyclization were further diversified via Pd-catalyzed cross-coupling reactions.
5. Palladium-catalyzed regioselective [3+2] annulation of internal alkynes and iodo-pyranoquinolines with concomitant ring opening: Efficient approach for the synthesis of pyrrolo[1,2-a]quinolines and acridones
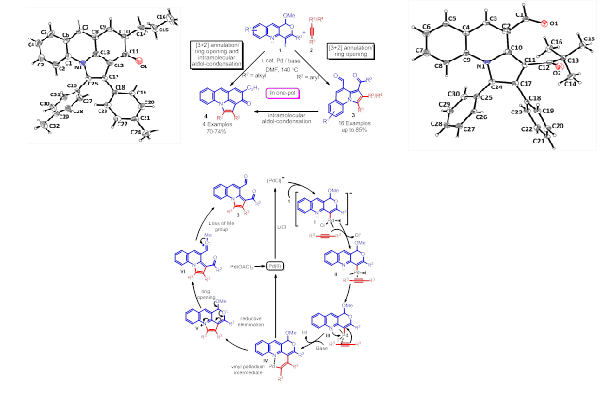
Org. Lett. 2012, 14, 5184–5187
A regioselective tandem synthesis of highly functionalized pyrrolo[1,2-a]quinolines has been developed through a novel strategy by palladium-catalyzed [3+2] annulation of iodo-pyranoquinolines and internal alkynes with subsequent ring opening. This chemistry was successfully extended for the synthesis of diverse pharmaceutically important pyrrolo-acridinone via [3+2] annulations/ring opening and successive intramolecular cross-aldol condensation. It is noteworthy, that unsymmetrical internal alkynes containing propargyl alcoholic group, selectively afforded single isomer. Further investigation of the scope and synthetic applications of the present strategy are currently underway and will be reported in due course.
6. Palladium-catalyzed sequential sonogashira/suzuki coupling and concomitant cyclization: A concise tandem route to phenanthrenes and naphthothiophenes
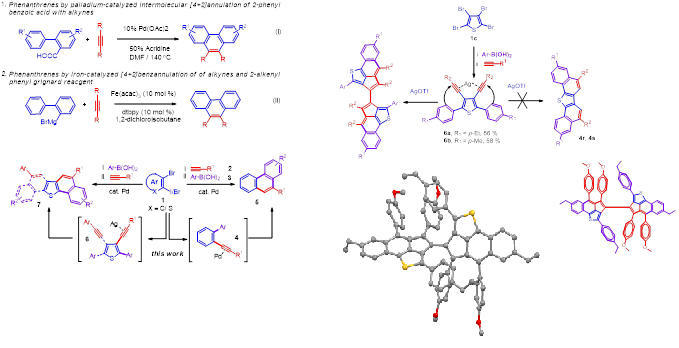
A concise synthetic pathway that enables the construction of phenanthrenes and naphthothiophenes using bench-top chemicals, ortho-dihaloarenes has been developed via palladium-catalyzed sequential Sonogashira/ Suzuki coupling reactions and subsequent electrophilic cyclization.
6. Palladium-catalyzed sequential sonogashira/suzuki coupling and concomitant cyclization: A concise tandem route to phenanthrenes and naphthothiophenes

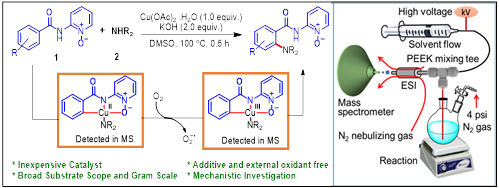
Copper-mediated highly efficient direct ortho C–H amination of arenes has been accomplished under external oxidant and additive free condition. The use of free primary and secondary amines as aminating agents makes the strategy more effective. The reaction tolerates a wide range of functional groups. Using online electrospray ionization mass spectrometry (ESI-MS), transient intermediates including copper complexes in different oxidation states were captured that implicates the intriguing possibility of two pathways: (a) CuIII-CuI and (b) CuII-Cu0. DFT calculations, in the current C–H amination reaction shows that the CuIII intermediate so formed could harness the exergonicity of CuIII-CuI reductive elimination, suggesting it to be more favorable pathway. The results provide guiding principles to design a catalytic cycle to explore the mechanism of this transformation.
8. Development of Diacetylene Based Colorimetric Radiation Sensors for Blood Irradiator Dosimetry and Colorimetric Quantification of Narrow Band UVB Radiation Doses in Phototherapy